Utilization of ExVive™ human liver tissues for the evaluation of valproic acid-induced liver injury
Publication Summary:
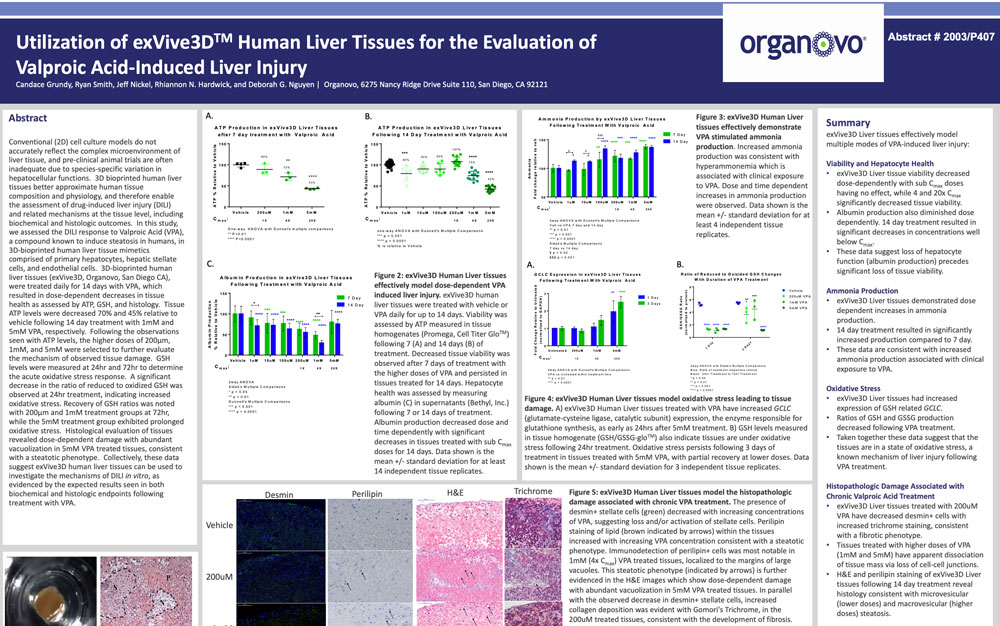
Conventional (2D) cell culture models do not accurately reflect the complex microenvironment of liver tissue, and preclinical animal trials are often inadequate due to species-specific variation in hepatocellular functions. 3D bioprinted human liver tissues better approximate human tissue composition and physiology, and therefore enable the assessment of drug-induced liver injury (DILI) and related mechanisms at the tissue level, including biochemical and histologic outcomes. In this study, we assessed the DILI response to Valproic Acid (VPA), a compound known to induce steatosis in humans, in 3D-bioprinted human liver tissue mimetics comprised of primary hepatocytes, hepatic stellate cells, and endothelial cells. 3D-bioprinted human liver tissues (ExVive™, Organovo, San Diego CA), were treated daily for 14 days with VPA, which resulted in dose-dependent decreases in tissue health as assessed by ATP, GSH, and histology. Tissue ATP levels were decreased 70% and 45% relative to vehicle following 14 day treatment with 1 mM and 5 mM VPA, respectively. Following the observations seen with ATP levels, the higher doses of 200µm, 1 mM, and 5 mM were selected to further evaluate the mechanism of observed tissue damage. GSH levels were measured at 24 hr and 72 hr to determine the acute oxidative stress response. A significant decrease in the ratio of reduced to oxidized GSH was observed at 24 hr treatment, indicating increased oxidative stress. Recovery of GSH ratios was noted with 200µm and 1 mM treatment groups at 72 hr, while the 5 mM treatment group exhibited prolonged oxidative stress. Histological evaluation of tissues revealed dose-dependent damage with abundant vacuolization in 5 mM VPA treated tissues, consistent with a steatotic phenotype. Collectively, these data suggest ExVive human liver tissues can be used to investigate the mechanisms of DILI in vitro, as evidenced by the expected results seen in both biochemical and histologic endpoints following treatment with VPA.
View Publication