Dear Shareholders
We are a biotechnology company pioneering the development of 3D bioprinted tissues aimed at treating a range of serious adult and pediatric liver diseases.

Change and What’s Ahead
It has been an exhilarating first year for me as Organovo’s CEO. We have evolved the way we define ourselves and how we bring the value of our platform technology to customers and future patients. We are harnessing our remarkable ability to 3D bioprint tissues with the primary goal of implanting our functioning tissues into pediatric and adult patients to treat a range of liver diseases. For these patients, treatment options are very limited, and an organ transplant is often the only path back to better health.
Fiscal 2018 was an important transition year, as we demonstrated preclinical proof-of-concept data for our NovoTissues® liver implant in animal models for Alpha-1-antitrypsin deficiency (“A1AT”). A1AT is our lead therapeutic indication, representing just one area in a group of rare, often fatal diseases known as inborn errors of metabolism (“IEMs”). We wrapped up the year by achieving orphan drug designation from the FDA for this first Investigational New Drug (“IND”)-track program. We aim to work closely with the FDA and our key advisers to confirm the scientific validation course we’ll need to follow for a successful pre-IND meeting for A1AT in order to commence IND-enabling studies in the near-term. We also began new animal model studies in a second therapeutic area known as Type 1 Tyrosinemia, and have presented early evidence showing good retention and functionality of our tissues, and improved survival rates in established animal models for this serious condition. As we look ahead, we’ll likely pursue orphan designation with the FDA for this second indication in fiscal 2019, with the objective of ending the year with two liver therapeutic tissue programs on track for an IND targeted for calendar 2020.

Our research programs will continue to explore additional liver disease areas including other inborn errors of metabolism, and we’ll also begin exploring the large market involving “acute on chronic” liver failure. When taking all these together, we estimate that the unique benefits of bridging patients to transplant or providing a therapeutic alternative to transplant could create an addressable peak global sales opportunity of more than $4 billion. The A1AT treatment alone has the potential to approach $1 billion in peak sales.
Using the same liver platform today, we’re also aiming to build immediate value by providing access to our technology platform for partners, collaborators and clients. Our foundational ability to characterize specialized human cells and build robust, functional human tissues allows us to create custom disease models that mimic non-alcoholic steatohepatitis (“NASH”) conditions. We believe our platform represents the only comprehensive, non-clinical way to investigate key aspects of drug efficacy and safety utilizing histology, which is the gold standard of diagnosing and measuring response in NASH. Because of our ability to create a disease testing capability, which we believe will be predictive of real human outcomes, we seek to provide “patient on a plate” results in a fraction of the time and costs of conducting human clinical trials.
Along with our early adopters, we’re pioneering a new path in this dynamic space of drug discovery and development, and we believe clients will turn to us to explore solutions across the R&D spectrum from novel drug targeting research, to comprehensive lead candidate profiling, to evaluating the competitive advantage aspects of drugs already in the clinic. Ultimately, the stream of revenues generated through this access provides scientific validation and financial support for our therapeutics pathway, and also has the potential to generate new pipeline ideas and capabilities.
Liver Therapeutic Tissue Pipeline
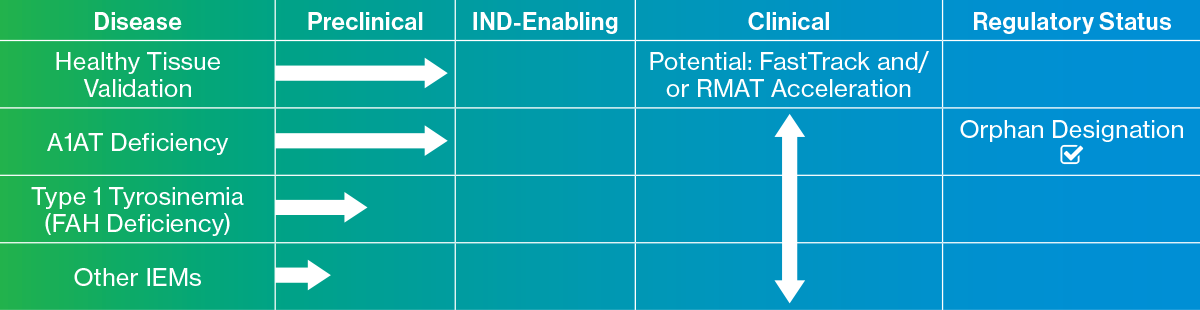

Creating long-term value
As we continue to demonstrate the remarkable utility of our 3D bioprinted tissues, we are focused on critical advances to medicine that our platform may provide, spanning our current support of NASH R&D through to a range of potentially breakthrough clinical applications enabled by our NovoTissues platform. We believe our technology has the potential to transform the care of patients with debilitating and often fatal liver diseases.
These key business and scientific objectives are aligned with strong financial discipline and the prudent management of our capital resources. We’ll continue to tightly manage our operating costs and streamline our operations to focus on our existing commercial opportunities and therapeutics research program. By leveraging our stream of near-term revenues, we hope to reduce the need for other methods of financing our therapeutics path to the clinic.
I thank my colleagues for their dedication and hard work during a busy and transformative year. I am grateful for the support of our clients, partners and stockholders. We enter fiscal 2019 with many important milestones in sight, for what is sure to be another exciting year for Organovo.
 Taylor J. Crouch
President and Chief Executive Officer
June 2018
Taylor J. Crouch
President and Chief Executive Officer
June 2018
